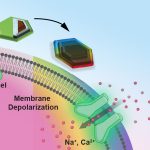
Lab-grown ‘tiny hearts’ bring hope for children and adults with genetic heart disease
Scientists from QIMR Berghofer’s Cardiac Bioengineering Lab have developed lab-grown, three-dimensional heart tissues known as cardiac organoids that mimic the…
Fast Four Quiz: Precision Medicine in Cancer
Bacteria ‘nanowires’ could help scientists develop green electronics
Engineered protein filaments originally produced by bacteria have been modified by scientists…
Nuisance Seaweed Found to Produce Compounds with Biomedical Potential
A seaweed considered a threat to the healthy growth of coral reefs…
How problems with an Alzheimer’s protein can jam up traffic in the brain
Materials move smoothly through the brain cells of a fruit fly larvae (left).…
A New Protein Target for Controlling Diabetes
Researchers at the University of California, San Diego School of Medicine have identified a previously…
Research finds key gene for regenerating cells after heart attack
UT Southwestern Medical Center researchers have pinpointed a molecular mechanism needed to unleash the heart’s ability to regenerate, a critical…
Sign Up for Free
Subscribe to our newsletter and don't miss out on our programs, webinars and trainings.
Aligned peptide ‘noodles’ could enable lab-grown biological tissues
A team of chemists and bioengineers at Rice University and the University of Houston have achieved a significant milestone in…
Your one-stop resource for medical news and education.
Nuisance Seaweed Found to Produce Compounds with Biomedical Potential
A seaweed considered a threat to the healthy growth of coral reefs in Hawaii may possess the ability to produce…
Nuisance Seaweed Found to Produce Compounds with Biomedical Potential
A seaweed considered a threat to the healthy growth of coral reefs in Hawaii may possess the ability to produce…
New tools used to identify childhood cancer genes
Using a new computational strategy, researchers at UT Southwestern Medical Center have identified 29 genetic…
Cotton-Based Hybrid Biofuel Cell Could Power Implantable Medical Devices
A glucose-powered biofuel cell that uses electrodes made from cotton fiber could someday help power…
New 3D printer uses rays of light to shape objects, transform product design
A new 3D printer uses light to transform gooey liquids into complex solid objects in…
Lab-grown ‘tiny hearts’ bring hope for children and adults with genetic heart disease
Scientists from QIMR Berghofer’s Cardiac Bioengineering Lab have developed lab-grown, three-dimensional heart tissues known as…
Diver-Operated Microscope Brings Hidden Coral Biology into Focus
The intricate, hidden processes that sustain coral life are being revealed through a new microscope…
A fungal origin for coveted lac pigment
The colourful pigment extracted from the lac insect may actually be produced by a symbiotic…
Combination approach could overcome treatment resistance in deadly breast cancer
QIMR Berghofer-led research in collaboration with Australian oncology company, Kazia Therapeutics, has found that combining…
Tailored brain stimulation treatment results give new hope for people with depression
Medical researchers at QIMR Berghofer have achieved a significant milestone in the treatment of depression,…
Game-Changer in Emergency Medicine: New AI Test Flags Sepsis Hours Before Symptoms Worsen
When the body's reaction to an infection goes awry, it can result in sepsis, a…
Perfumes and lotions disrupt how body protects itself from indoor air pollutants
Fragrances and lotions don't just change the way people smell, they actively alter the indoor…
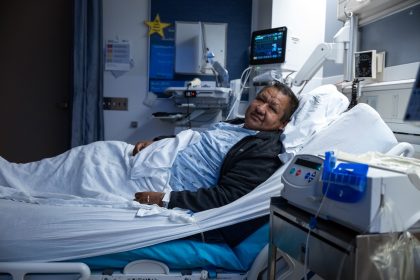
Medical Milestone: Surgeons Perform First-Ever Human Bladder Transplant
In a groundbreaking medical achievement, surgeons at the University of California, Los Angeles (UCLA) Health…